Hydrocarbons, any class of organic compounds consisting only of the elements carbon (C) and hydrogen (H). The carbon atoms are joined together to form the framework of the compound, and the hydrogen atoms are attached to them in many different configurations. Hydrocarbons are the main components of oil and natural gas.
Many hydrocarbons are found in nature. In addition to making up fossil fuels, they are found in trees and plants, for example, in the form of pigments called carotenoids in carrots and green leaves. More than 98% of natural crude rubber is a hydrocarbon polymer, a chain-like molecule consisting of many linked units. The structure and chemical properties of individual hydrocarbons depend greatly on the type of chemical bonds that hold the atoms of their constituent molecules together.
Aliphatic hydrocarbons are divided into three main groups based on the type of bonds they contain: alkanes, alkenes and alkynes. Alkanes have only single bonds, alkenes contain carbon-carbon double bonds, and alkynes contain carbon-carbon triple bonds. Aromatic hydrocarbons are those that are more stable than their Lewis structure would suggest; that is, they have "special stability. They are classified as either arenes, which contain a benzene ring as a structural unit, or non-benzene aromatic hydrocarbons, which have special stability but lack a benzene ring as a structural unit.
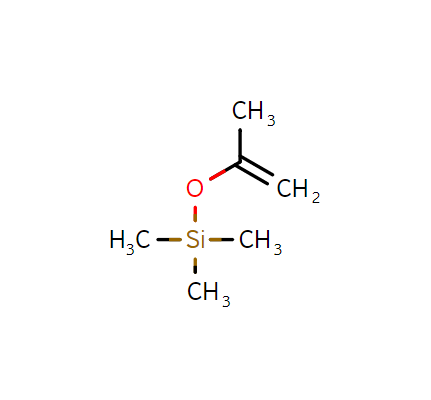
Isopropenoxytrimethylsilane, CAS:1833-53-0
This classification of hydrocarbons helps to relate structural features to properties, but it is not necessary to assign specific substances to a single category. In fact, a molecule usually contains two or more structural units characteristic of the hydrocarbon group. For example, a molecule that contains both a carbon-carbon triple bond and a benzene ring will exhibit some of the properties of an alkyne and the properties of an aromatic hydrocarbon.
Alkanes
Alkanes are hydrocarbons in which all bonds are single bonds and whose molecular formulae satisfy the general expression C n H 2 n + 2 (where n is an integer). The carbon is s p 3 hybridized and each C-C and C-H bond is a σ-bond. Methane (CH 4), ethane (C 2 H 6 ), and propane (C 3 H 8 ) are the first three members of the series in increasing order of carbon atom number.
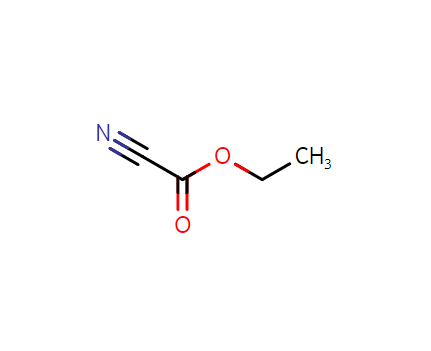
2-ethoxy-2-oxoacetonitrile, CAS:623-49-4
Cycloalkanes
Countless organic compounds are known in which a series of carbon atoms are not joined in a chain but closed to form a ring. A saturated hydrocarbon containing a ring is called a cycloalkane. The general formula is C n H 2 n (n is an integer greater than 2), and they have two fewer hydrogen atoms than alkanes with the same number of carbon atoms. Cyclopropane (C 3 H 6 ) is the smallest cyclic alkane.
Olefins and alkynes
Olefins (also called olefins) and alkynes (also called acetylenes) belong to the group of unsaturated aliphatic hydrocarbons. Olefins are hydrocarbons containing carbon-carbon double bonds, while alkynes have carbon-carbon triple bonds. Olefins are characterized by the general formula C n H 2 n and alkynes are characterized by C n H 2 n - 2. Ethylene (C 2 H 4 ) is the simplest olefin and acetylene (C 2 H 2 ) is the simplest alkyne.
Hydrocarbons are the main components of oil and natural gas, and they are used as fuels and lubricants and as raw materials for the production of plastics, fibers, rubber, solvents, explosives, and industrial chemicals that are important raw materials in our lives.