Targeted Protein Degradation (TPD) mainly involves the degradation of target proteins through the ubiquitin-proteasome and lysosome systems. There are nearly ten different technological routes within TPD, among which molecular glue and PROTAC are the fastest growing ones.
BMS's molecular glue Lenalidomide has annual sales of US$12.891 billion. Arvinas' PROTAC molecule ARV-471 has entered Phase III clinical trials, as lysosome-based degradation is relatively new and still in the preclinical stage.
At present, conventional targets are exhausted and new targets are difficult to discover in drug development. TPD technology provides a new path for drug development and expands the range of targetable proteins, and is one of the most promising technologies for future development. Next, let’s introduce PROTAC and molecular glue.
01 Differences between PROTAC and Molecular Glue
Proteolysis Targeting Chimeras(PROTACs)and molecular glue are two technologies rapidly developed in recent years in targeted protein degradation. They result in protein degradation for treatment of diseases through different mechanisms, and both methods have the potential to change the traditional way of drug development.
① Structural Differences: PROTACs typically consist of three parts: a ligand of a target protein, a linker, and an E3 ligase-binding warhead. This structural design allows PROTAC molecules to interact with both the target protein and the E3 ligase simultaneously.
Whereas, molecular glue is usually a single small molecule that alters the conformation of the target protein or E3 ubiquitin ligase, facilitating their interaction without requiring a specific linker or high-affinity ligand for the target protein (Figure 1).
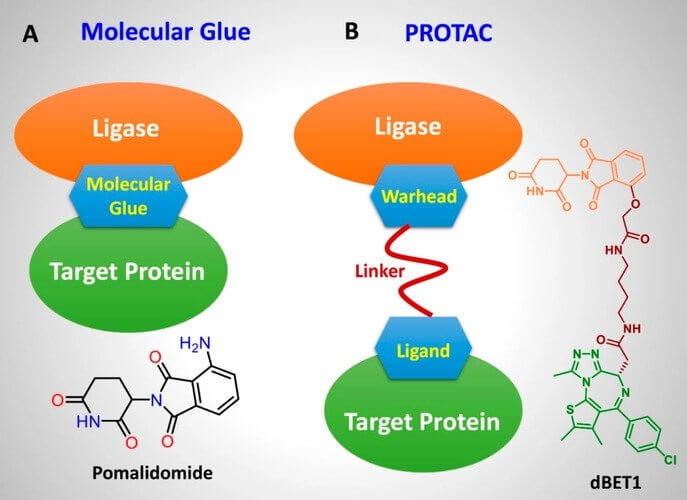
Figure 1. Mode of action and structural features of molecular glue and PROTAC.
② Mechanism Differences: The main difference between PROTACs and molecular glue lies in their mechanisms of action. PROTACs induce TPD by connecting the target protein with the ligand for E3 ubiquitin ligase, allowing the ubiquitin attached to the target protein and subsequent degradation in the proteasome. This approach can completely eliminate pathological proteins and counteract their disease activity. In contrast, molecular glue forms stable complexes through stabilization and affinity interactions with the pathological protein, inhibiting its activity or promoting the degradation of the target protein.
③ Efficiency Differences: PROTACs can achieve selective degradation of the target protein, and their efficiency depends on the affinity between the PROTAC molecule and the target protein as well as the E3 ligase. Unlike conventional enzyme inhibitors or receptor blockers, PROTACs not only inhibit protein function but also reduce the amount of the protein.
Similarly, molecular glue can induce TPD by directly binding the target protein to the E3 ligase. The efficiency of molecular glue also depends on the affinity between the molecule and the target protein as well as the E3 ligase (Table 1).
|
Molecular glue |
PROTAC |
|
|
Mechanism |
Binds E3 or target protein induces PPI |
Binds target and E3 |
|
Target protein (POI) |
To be determined |
Predictable |
|
Discovery strategy |
Historically serendipitous discovery |
Rational design |
|
Feature |
Monovalent |
Bivalent |
|
Linker |
Without linker |
With linker |
|
Molecular weight |
Lower |
Higher |
|
Lipinski’s rule of five |
Typically within |
Beyond |
|
Binding pocket in the target protein |
Nonessential |
Required |
|
Binding affinity |
Weaker binding affinities for either E3 ligase |
Stonger binding affinities for either E3 ligase |
Table 1. Comparison of Molecular Glues and PROTACs
02 Frontier Developments of PROTAC and Molecular Glue
PROTACs have been successfully employed in research of various protein-related diseases and have shown strong therapeutic effects in clinical trials. Examples include ARV-471 targeting estrogen receptor and MT-802 targeting BTK, etc. However, no related products of PROTAC drugs have been approved globally. Some overseas companies, such as Arvinas, C4 Therapeutics, Kymera Therapeutics, and Nurix, have made significant progress.
With Arvinas' two candidates, ARV-110 and ARV-471 obtaining positive clinical data, this field has experienced vigorous growth in the past two years. Currently, multiple PROTAC drugs have entered clinical stages, with targets including AR, ER, BCL-XL, IKZF1/3, STAT3, BTK, TRK, BRD9, etc. In addition, Kymera Therapeutics, C4 Therapeutics, and Nurix Therapeutics have molecules in clinical stages. Chinese pharmaceutical companies like Lynk Pharmaceuticals, Kintor Pharmaceutical, Haisco Pharmaceutical, Meizer Pharma, Cullgen, Hinova Pharmaceuticals, and Seed Therapeutics, a subsidiary of BeyondSpring Pharmaceuticals, have already deployed in the PROTAC field. Among them, Lynk Pharmaceuticals, Kintor Pharmaceutical, and Haisco Pharmaceutical have PROTAC molecules in clinical stages (Table 2).
|
PROTAC |
Target |
Indications |
Phase |
Company |
|
ARV-471 |
Estrogen receptor (ER) |
Breast cancer (ER+/HER2-Breast Cancer) |
Phase III |
Arvinas |
|
ARV-110 |
Androgen receptor (AR) |
Metastatic castration-resistant prostate cancer (mCRPC) |
Phase II |
Arvinas |
|
ARV-766 |
Androgen receptor (AR) |
Metastatic castration-resistant prostate cancer (mCRPC) |
Phase II |
Arvinas |
|
KT-474 |
Interleukin-1 receptor-associated kinase 4 (IRAK-4) |
Atopic dermatitis, hidradenitis suppurativa, rheumatoid arthritis |
Phase II |
Kymera Therapeutics |
|
LNK01001 |
- |
Rheumatoid arthritis, atopic dermatitis, ankylosing spondylitis |
Phase II |
Lynk Pharmaceuticals |
|
GT20029 |
AR |
Androgenic alopecia |
Phase II |
Lynk Pharmaceuticals |
|
CFT7455 |
IKZF1/3 |
Relapsed/refractory non-Hodgkin lymphoma or multiple myeloma |
Phase I/ II |
C4 Therapeutics |
|
CFT1946 |
BRAF V600 |
BRAF V600 mutated solid tumors |
Phase I/ II |
C4 Therapeutics |
|
NX-2127 |
BIK + IKZF |
B cell malignancies |
Phase I |
Nurix Therapeutics |
|
NX-5948 |
BTK |
B cell malignancies and autoimmune diseases |
Phase I |
Nurix Therapeutics |
|
UNK01002 |
- |
Blood cancer |
Phase I |
Lynk Pharmaceuticals |
|
LNK01003 |
- |
Immunity, inflammation |
Phase I |
Lynk Pharmaceuticals |
|
HSK29116 |
BTK |
B cell malignancies |
Phase I |
Haisco Pharmaceutical |
|
MZ-001 |
BTK |
B-cell malignancies and autoimmune diseases |
Phase I |
Meizer Pharma |
|
HP518 |
AR |
mCRPC that fails standard therapy |
Phase I |
Hinova Pharmaceuticals |
|
CFT8919 |
EGFR L858R |
Resistant EGFR-mutated non-small cell lung cancer (NSCLC) |
Phase I |
C4 Therapeutics |
|
CFT8634 |
BRD9 |
Synovial sarcoma and smarcb1-deficient solid tumors |
Phase I |
C4 Therapeutics |
|
KYM-001 |
KYM-001 |
MYD88 mutated B-cell lymphoma |
IND |
Kymera Therapeutics |
|
CG416 |
Tyrosine kinase receptor (TRK) |
- |
IND |
Cullgen |
|
CG428 |
TRK |
- |
Preclinical |
Cullgen |
|
CG001419 |
TRK |
- |
Preclinical |
Cullgen |
|
HC-X029 |
AR-SV |
End-line treatment of mCRPC after failure of standard therapy |
Preclinical |
Hinova Pharmaceuticals |
|
HC-X035 |
Src-homology 2 domain-containing protein tyrosine phosphatase (SHP2) |
KRAS mutated cancer |
Preclinical |
Hinova Pharmaceuticals |
|
AR-V7 |
AR-FL & AR-V7 |
Metastatic castration-resistant prostate cancer (mCRPC) |
Preclinical |
Arvinas |
Table 2. PROTACs in Clinical Trials
Another relatively new therapy, molecular glue is still in early research and development.It has shown therapeutic potential in some specific cases, and currently approved molecular glues mainly consist of immunomodulators, namely thalidomide, lenalidomide, and pomalidomide, which are used to treat multiple myeloma and myelodysplastic syndrome, etc. These three molecular glue degraders have molecular weights below 300 Da and degrade target proteins including the transcription factor IKZF1/3 by recruiting the E3 ubiquitin ligase CRBN. Additionally, thalidomide analogs are commonly used as ligands for the E3 ubiquitin ligase CRBN in many PROTAC molecules. For instance, the E3 ligand for ARV-471, which has entered Phase III clinical trials, is based on (R)-thalidomide.
Apart from three approved ones, there are currently over twenty molecular glue degraders in clinical or preclinical stages. Overseas companies such as BMS, C4 Therapeutics, Nurix, and Monte Rosa Therapeutics, etc. have made significant progress. Undoubtedly, BMS holds a leading position in this field. The molecular glue pipeline mainly comes from the acquisition of Celgene products in 2019, including Lenalidomide, CC-92480, CC-99282, CC-220, and others. Nurix and C4 Therapeutics also have molecular glue degraders in clinical trials. Preclinical molecular glue development companies include Monte Rosa Therapeutics, Ambagon Therapeutics which targets degradation of intrinsically disordered proteins, and Ranok Therapeutics, which was the first to report a BRD4 degrader. The targets of molecular glue degraders include IKZF1/2/3, RBM39, GSPT1, CK1α, BCL6, Cyclin K, NEK7, primarily for hematologic malignancies, and some solid tumors. Chinese companies like InnoCare Pharma and Gluetacs Therapeutics have also conducted clinical research on molecular glues. InnoCare Pharma’s ICP-490 has already in Phase II clinical trials for multiple myeloma (Table 3).
|
Molecular glue |
Target |
Indications |
Phase |
Company |
|
Lenalidomide |
1KZF1/3 |
Multiple myeloma |
Approved |
BMS/Celgene |
|
Thalidomide |
1KZF1/3 |
Multiple myeloma |
Approved |
BMS/Celgene |
|
Pomalidomide |
1KZF1/3 |
Multiple myeloma |
Approved |
BMS/Celgene |
|
CC-92480 |
1KZF1/3 |
Multiple myeloma |
Phase l/ II/ III |
BMS/Celgene |
|
CC-220 |
FP91/98;1KZF1 |
Solid tumors |
Phase l/ II/ III |
BMS/Celgene |
|
E7820 |
RBM39 |
Acute myeloid leukemia |
Phase II |
Eisai |
|
ICP-490 |
1KZF1/3 |
Multiple myeloma |
Phase II |
InnoCare Pharma |
|
CC-99282 |
1KZF1/3 |
Lymphoma |
Phase I/ II |
BMS/Celgene |
|
MRT-2359 |
GSPTI |
Lung cancer and other solid tumors |
Phase I/ II |
Monte Rosa |
|
CFT-7455 |
1KZF1/3 |
Multiple myeloma |
Phase I/ II |
C4 Therapeutics |
|
DKY-709 |
1KZF2 |
NSCLC, melanoma |
Phase l/ lb |
Novartis |
|
CC-90009 |
GSPTI |
Acute myeloid leukemia |
Phase I |
BMS/Celgene |
|
GT-919 |
1KZF1/3 |
Multiple myeloma |
Phase I |
Gluetacs Therapeutics |
|
BTX-1188 |
GSPT1; IKZF1/3 |
Hematoma, solid tumor |
Phase I |
BioTheryX |
|
BAY-2666605 |
PDE3A/SLFN12 |
Melanoma |
Phase I |
Bayer |
|
TMX-4116 |
CK1α |
Multiple myeloma |
Preclinical |
Dana-Farber |
|
(R)-CR8 |
Cyclin K |
Lung cancer and other solid tumors |
Preclinical |
Broad Institute |
|
BI-3802 |
Bcl-6 |
Lymphoma |
Preclinical |
Boehringer Ingelheim |
|
NRX-252114 |
β-Catenin mutant |
Lung cancer |
Preclinical |
Nurix Therapeutics |
|
MGD molecule |
NEK7 |
Inflammatory diseases |
Preclinical |
Monte Rosa |
Table 3. Molecular glues in Clinical Trials
03 Future Trends of PROTAC and Molecular Glue
Though there are no PROTAC drugs on the market, several candidates demonstrate preliminary clinical data with significant degradation of intracellular proteins and promising therapeutic effects.However, the validation process is gradual and requires larger sample sizes to confirm these findings. Due to its unique mechanism of action, PROTAC technology is attracting more and more biomedical innovators and entrepreneurs to compete on this new track. Through twenty years of development, PROTAC has broken through the rules of established medicines that have always been widely recognized, and opened a new chapter for the development of new drugs in the continuous exploration and advancement. It is expected that PROTAC technology will find even broader applications in future drug development. Meanwhile, as technology advances, the design and preparation processes of PROTAC are being more precise and controllable.
Currently, over 600 E3 ligases have been reported, but only five have been used for molecular glue-mediated degradation, namely CRBN, DDB1, β-TrCP, DCAF15 and SIAH1. The E3 ligase library still holds huge potential for further exploration, and the identification of new ligands for E3 ligases will expand our range of degradable target proteins. Additionally, there is a need for more exploration of the chemical space of molecular glue molecules. Most reported molecular glues so far are still highly similar to thalidomide and its derivatives. This would be indeed a considerable challenge for drug developers. We need to deepen our insights and understanding of protein-protein interaction interface, make more reasonable structure-guided molecular glue designs, in order to truly promote molecular glues into clinical applications and help treat a wider range of diseases.
Source:
[1] Dong, G.; Ding, Y.; He, S.; Sheng, C. Molecular Glues for Targeted Protein Degradation: From Serendipity to Rational Discovery. J Med Chem. 2021, 64 (15), 10606-10620.
[2] Zhao, L.; Zhao, J.; Zhong K.; Tong, A.; Jia, D. Targeted protein degradation: mechanisms, strategies and application. Signal Transduct Target Ther. 2022, 7 (1), 113.
[3] CAS Insights. Targeted Protein Degradation and Induced Proximity: Molecular Glues Landscape in Drug Discovery. https://www.cas.org/resources/cas-insights/drug-discovery/targeted-protein-induced-proximity (accessed 2024-01-31).
Please contact us to remove any infringement.